Single Blind Studies
Example of a single blind study for example imagine that researchers are doing a study to determine if a certain type of medication causes people to feel more alert.
Single blind studies. A single blind study can help prevent this or minimize the effects of such demand characteristics. Term is thus unhelpful without clarification. Uncontrolled studies have suggested a beneficial effect of periodontal treatment on metabolic control of insulin dependent diabetes mellitus iddm.
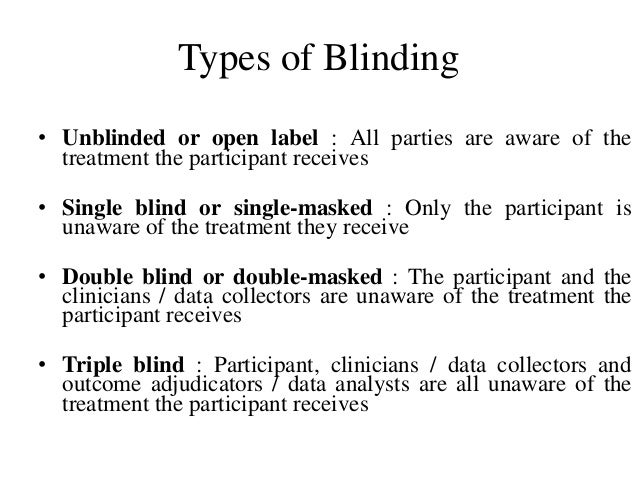
A survey of physicians and a review of textbooks and reports revealed numerous interpretations of the designation double blind 2 of key importance in both single and double blind trials is whether the outcome assessor is blinded. However there are situations where creating such ignorance might be impossible or unethical and in others it might be advisable for more than the participants to be kept unaware of the test conditions. Most often single blind studies blind patients to their treatment allocation double blind studies blind both patients and researchers to treatment allocations and triple blinded studies blind patients researcher and some other third party such as a monitoring committee to treatment allocations.
In a triple blind study the assignment is hidden not only from participants and experimenters but also from the researchers analyzing the data. This is done to reduce the risk of errors since some participants might produce spurious results if they know that they are taking the placebo or medication. A technique for eliminating subjective bias as the placebo effect from the test results.
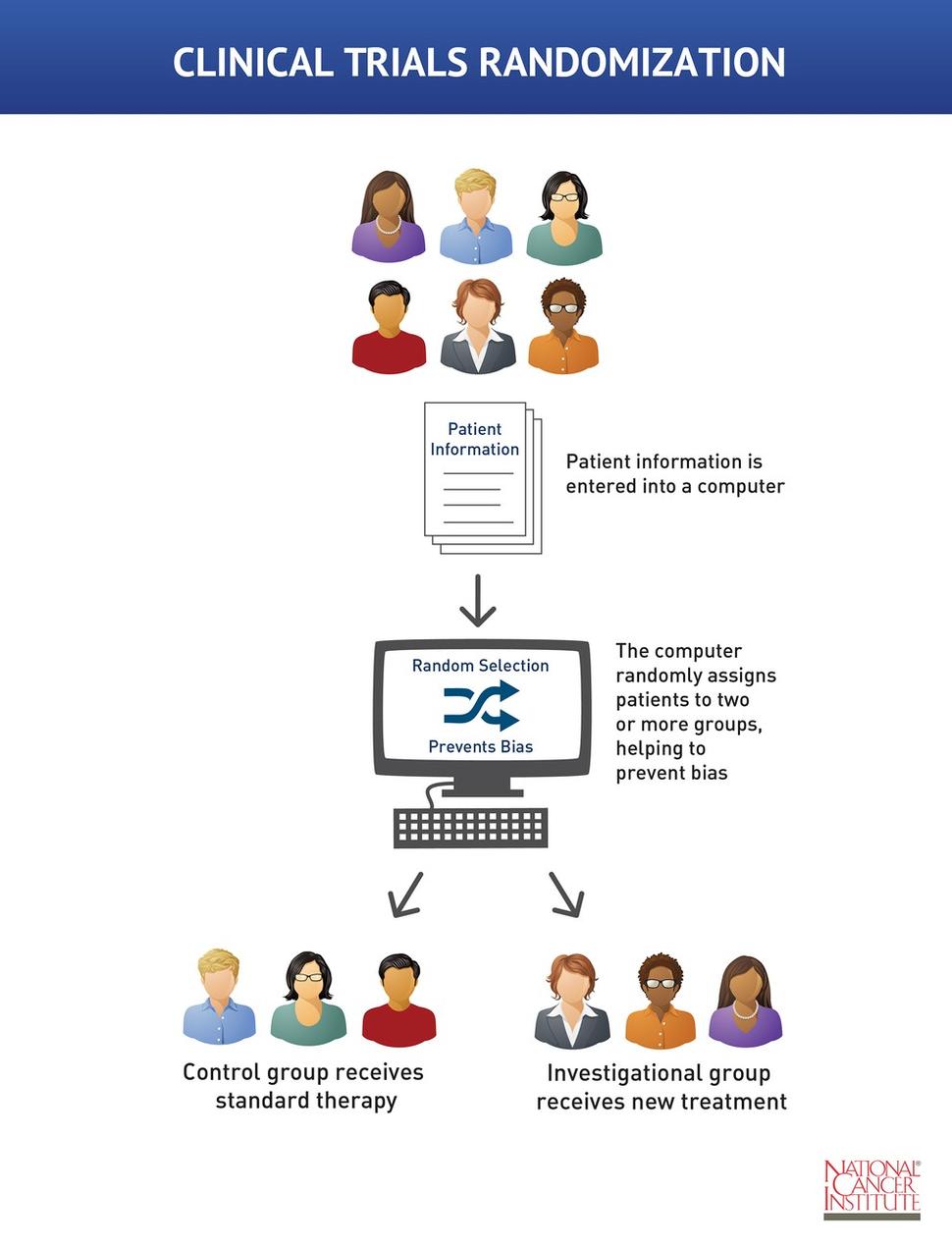
In a single blind study the participants in the clinical trial do not know if they are receiving the placebo or the real treatment. Double blind trials are just as confusing as single blind trials. There were three significant differences in the behaviour of the conference s single vs double blind review groups.
Reviewer bias in single vs double blind conditions. We therefore conducted controlled single blind studies using current metabolic status indicators in iddm subjects free of significant complications ot. In a double blind study both participants and experimenters are blinded.
Single blind definition of or relating to an experiment or clinical trial in which the researchers but not the subjects know which subjects are receiving the active medication or treatment and which are not. The prospective randomised single blind study was conducted at ordu university training and research hospital ordu turkey from january 1 to june 30 2015 and comprised boys aged 6 12 years who were scheduled to undergo circumcision operation. What is the difference between single blind double blind and triple blind studies.